News & Articles

Treating Leukemia with CAR T-cell Therapy

Parkway Cancer Centre (PCC) is the first private medical centre in Singapore to offer Chimeric Antigen Receptor (CAR) T-cell Therapy, a new cancer treatment that is making breakthroughs in the treatment of complex blood cancers. In this issue of HealthNews, Dr Dawn Mya explains the role of CAR T-cell Therapy in the management of leukemia.
CAR T-cell Therapy has recently emerged as the latest treatment approved to be delivered commercially by the U.S. Food and Drug Administration (FDA) as well as the Singapore Health Sciences Authority (HSA) under a new Cell, Tissue and Gene Therapy Products (CTGTP) regulatory framework.
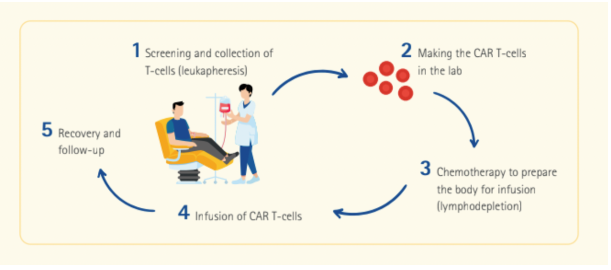
The treatment, which is a form of immunotherapy, involves extracting a type of white blood cells in the immune system called T-cells from the patient’s blood, and modifying them in a laboratory setting to enable them to recognise certain targets on certain cancers.
The modified T-cells—or CAR T-cells—are then reinfused into the patient to detect cancer cells and destroy the cancer by harnessing the body’s own immune response. By reengineering the body’s natural defense, the immune system is able to eradicate the cancer from within, with unrivalled advantages to standard treatment modalities.
Treating leukemia with CAR T-cell Therapy
CAR T-cell Therapy is currently approved for use in the treatment of B-cell Acute Lymphoblastic Leukemia (B-ALL), leukemia in paediatric patients and young adults up to the age of 25, and relapsed and refractory cases.
Although CAR T-cell Therapy is a novel treatment that has only recently been approved for commercial use, it has shown promising outcomes for the treatment of leukemias. The remission rate for high-risk patients with relapsed or refractory B-ALL is an impressive >82% with CAR T-cell Therapy, a stark comparison to a remission rate <10% when given standard treatment. Patients who are responsive to treatment also continue to be stable and free of disease, with 7 out of 10 patients surviving after 2 years.
As CAR T-cell Therapy is a highly specialised treatment for selected patients with specific indications and conditions, the eligibility for CAR T-cell Therapy is assessed based on patient factors and disease factors:
Patient factors
- Overall fitness to receive lymphodepleting chemotherapy (lymphodepletion) to prepare the body to receive CAR T-cells
- Adequate organ functions (heart, lungs, kidney, liver) to be able to handle treatment
- No ongoing serious infections (bacterial, viral, fungal, HIV, Hepatitis B/C)
- No concurrent cancers
- No active autoimmune disorders
- Age limit of up to 25 years
Disease factors (for patients with B-ALL only)
- Refractory cases
- Second relapse
- Relapse post-allogeneic stem cell transplant
- For Philadelphia chromosome positive B-ALL, patient must have failed or be intolerant to 2 lines of targeted therapy
- No active leukemia in the central nervous system (brain and spinal space)
Patients who are eligible to receive CAR T-cell Therapy may also undergo allogeneic stem cell transplant subsequently if deemed necessary, and vice versa.
CAR T-cell Therapy: How It Works
Side effects and complications
Like any other treatment, CAR T-cell Therapy can trigger a range of side effects and potential complications.
A common side effect of CAR T-cell Therapy is Cytokine Release Syndrome (CRS), which is a multisystemic disease resulting from the overwhelming immune response against cancer cells. Side effects of CRS may range from fever to low blood pressure, difficulty breathing and low oxygen levels.
Another known side effect of CAR T-cell Therapy is immune effector cell-associated neurotoxicity syndrome (ICANS), which affects the central nervous system of the patient, resulting in altered mental status, seizures and other neurological symptoms.
Tumour lysis syndrome may also occur in cases where many leukemic cells are present at the time of CAR T-cell infusion. This is caused by the release of high amounts of uric acid and phosphate, resulting in kidney failure and heart problems.
Other complications arising from CAR T-cell Therapy include low immunity levels and higher risk of infection. However, many of these side effects are well-recognised and can be managed by a highly trained, multidisciplinary clinical care team. To ensure continuity of care, patients will be monitored for efficacy, response and side effects for a period of time following treatment.
More revolutionary breakthroughs ahead
Despite its challenges, CAR T-cell Therapy offers major breakthroughs in the treatment of haematological malignancies, with high success rates in the treatment of leukemia, as well as other complex blood cancers. The treatment offers a significant survival advantage compared to conventional chemotherapy, especially for patients whose diseases have relapsed or previously failed to respond to standard treatment.
The high overall success rates in achieving remission with CAR T-cell Therapy is testament to the extensive, groundbreaking advances in medical research and technologies in the field of Haematology over the years. As cell therapies continue to advance and develop with research and time, there is much hope for CAR T-cell Therapy to carve more revolutionary breakthroughs in the treatment of blood cancers as well as non-haematological cancers in the future.
| POSTED IN | Cancer Treatments |
| TAGS | adult cancer, blood cancer, cancer awareness, cancer latest breakthrough, common side effects of cancer treatment, immunotherapy, leukaemia in children |
| READ MORE ABOUT | Acute Lymphoblastic Leukaemia (ALL) in Adults, Acute Lymphoblastic Leukaemia (ALL) in Children, Leukaemia |
| PUBLISHED | 01 May 2022 |
