News & Articles

CAR T-cell Therapy: The Horizon of Possibilities

Dr Dawn Mya, Senior Consultant, Haematology, discusses Chimeric Antigen Receptor (CAR) T-cell Therapy, one of the latest therapies that is paving the way for revolutionary change in the treatment of complex blood cancers.
Speaking at a webinar with the Philippine College of Hematology and Transfusion Medicine, Dr Mya explained how the emergence of novel therapies like CAR T-cell Therapy is making significant breakthroughs in the field of Haematology.
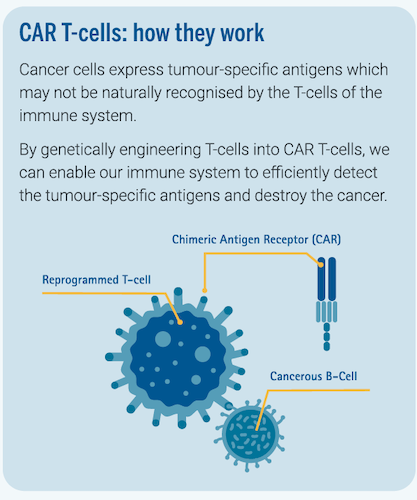
CAR T-cell Therapy is a revolutionary therapy that combines the concepts of cell therapy, living drugs, immunotherapy and targeted therapy to offer patients with blood cancer individualised and personalised treatment by harnessing the body’s natural immune system to fight cancer.
It involves extracting T-cells—a type of white blood cells in the immune system—from the patient’s blood and genetically modifying them in a laboratory setting to enable the T-cells to detect certain targets on the cancer cells. The modified T-cells—also known as CAR T-cells—are then reinfused into the patient to destroy the cancer cells.
Treating complex blood cancers
Anti-CD19 CAR T-cells are the very first CAR T-cells to be approved for clinical use by the U.S. Food and Drug Administration (FDA) as well as the Singapore Health Sciences Authority (HSA) under a new Cell, Tissue and Gene Therapy Products (CTGTP) regulatory framework. Singapore is the first country in Southeast Asia to offer the treatment1.
This exciting therapeutic development in the field of Haematology offers significant improvements in survival outcomes for the treatment of aggressive blood cancers such as B-cell Acute Lymphoblastic Leukaemia (B-cell ALL) and Diffuse Large B-cell Lymphoma (DLBCL), especially in relapsed or refractory settings.
For B-cell ALL, approximately 10–20% of patients have relapsed or refractory disease following first-line treatment. Less than 10% of these patients have long-term overall survival.
However, with CAR T-cell Therapy, the treatment outcome for B-cell ALL patients has significantly improved. Approximately 7 out of 10 paediatric and adolescent patients age 2–25 with relapsed or refractory B-cell ALL survive 2 years after treatment, with just a one-time infusion of CAR T-cells.
For DLBCL, approximately 40% of patients can have primary refractory disease or relapse following first-line treatment. This group of patients has a low overall survival rate of less than 20%. With CAR T-cell Therapy, the treatment outcome for relapsed or refractory DLBCL patients has significantly improved with an overall response rate of 52% and complete remission rate of 40%.
CAR T-cell Therapy is also challenging the role of salvage chemotherapy and autologous stem cell transplantation (SCT)—which is the standard of care in the treatment of relapsed and refractory DLBCL, with significantly improved outcomes.
The treatment journey
The treatment journey for CAR T-cell Therapy begins with patient selection to ensure the patient is suitable to receive treatment. This is an important process that is typically done by the patient’s primary haematologist.
Generally, eligible patients include:
- Children and young adult patients from 2–25 years old with B-cell ALL which is refractory to the first line treatment or which has relapsed after treatment including stem cell transplantation.
- Adults with DLBCL who have not benefited from at least two lines of standard treatment.
Patients will also be assessed for suitability based on their general health and disease status.
If the patient is deemed suitable for treatment, they will undergo a procedure called leukapheresis to extract T-cells from their blood. The T-cells are then transported to the laboratory to be genetically engineered into CAR T-cells.
As the process takes 2–3 weeks, some patients may require bridging chemotherapy to control their disease while waiting for the CAR T-cells to be infused.
A short chemotherapy cycle, called lymphodepleting chemotherapy, will also be administered a few days before the infusion of CAR T-cells. This is carried out to help create a favourable immune environment in the patient, so that the patient’s body is prepared to receive the CAR T-cells.
After infusion, the patient will be monitored for treatment response and any potential side effects.
Effectiveness and challenges
CAR T-cell Therapy is a high-risk therapy that comes with its challenges. Side effects of treatment include cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), which may develop after the infusion of CAR T-cells.
Fortunately, these challenges can be managed by a well-equipped clinical care team that is trained to monitor potential complications.
CAR T-cell Therapy remains a revolutionary treatment that is highly effective and offers improved life expectancy for the treatment of B-cell ALL and DLBCL. It offers patients hope in the event that their diseases have previously failed to respond to first-line treatment.
Current clinical trials on CAR T-cell Therapy are looking at how the revolutionary breakthroughs we have seen thus far with CAR T-cell Therapy can expand into the treatment of multiple myeloma and other subtypes of non-Hodgkin lymphoma, as well as challenge the role of conventional autologous SCT in relapsed and refractory settings.
In summary, the emergence of CAR T-cell Therapy brings hope to many patients with blood cancer in the future.
